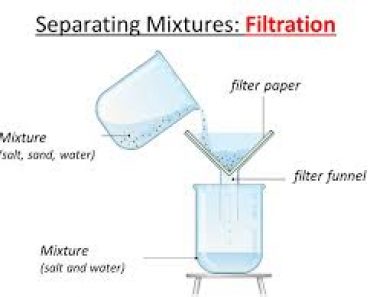
Separate Sand and Salt by Filtration
NAME OF EXPERIMENT : TO SEPARATE THE SAND AND COMMON SALT FROM MIXTURE OF THEM BY FILTRATION .
APPARATUS REQUIRED : Porcelain basin , funnel , filter paper , wire gauze , burner , test tubes etc.
CHEMICALS REQUIRED : Sand , Common Salt (NaCl) , Silver Nitrate (AgNO3) etc.
THEORY : The soluble and insoluble components of the mixture are separated by dissolving the soluble component in water followed by filtration . Sand and Common Salt are separated by dissolving salt in water and followed by filtration .
Filtration is the process of separating the water insoluble component of the solution by means of porous medium like filter paper or glass wool . The insoluble component remains in the filter paper as residue . The soluble component obtained is called filtrate . Filtrate contains Sodium Chloride solution and pure salt is obtained by washing of sand by water and pure salt is obtained by evaporation of filtrate .
Salt + Sand + water —-> Sand + Salt solution —> Filtration —> Salt Solution + Evaporation —> Salt
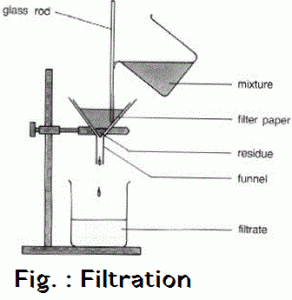
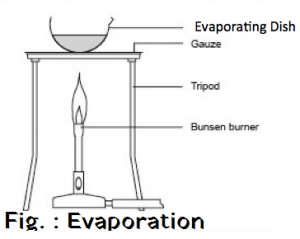
OBSERVATION :
PURITY TEST FOR SAND :
| Experiement | Observation | Inference |
|---|---|---|
| 1. Few cc of washing sand is taken into the test tubes and 1-2 drops of AgNO3 was added. | 1. Curdy white precipitate was obtained. | 1. Presence of common salt in sand ( Presence of CL- ) |
| 2. Few cc of washing sand is taken into the test tubes and 1-2 drops of AgNO3 was added. | 2. Curdy white precipitate was obtained. | 2. Presence of common salt in sand ( Presence of CL- ) |
| 1. Few cc of washing sand is taken into the test tubes and 1-2 drops of AgNO3 was added. | 1. Curdy white precipitate was not obtained. | 1. Presence of common salt in sand ( Absence of CL- ) |
TEST REACTION :
AgNO3 (aq.)+ NaCl (aq.) —> AgCl (s) + NaNO3 (aq.) (white ppt.)
RESULT : Hence , the mixture of sand and common salt was separated in pure and dry state .
CONCLUSION : Mixture of water soluble and insoluble substance can be filtered by filtration followed by evaporation .
Some useful links : Define Presentation software and write its features. | Grade 11 , Workbook, Worksheet, Cell, Cell Reference Short Notes | Grade 11


 - [Resolving the Adventure Not Found Error in For the King 2](#) - [Understanding the Purpose of the Hardwork Skill in For the King 2](#) Upon liberating the prisoner from the cart in The Resistance chapter, the world unfurls for exploration. Roam the area until you chance upon an overturned wagon distinct from the prisoner cart, nestled in the Foothills area of the map. Should the wagon remain elusive, lean on Vision Scrolls or Find Distance items, available in town shops, dropped by enemies, or carried by specific characters such as the Scholar. Employ these tools to meticulously scrutinize the Foothills. Continue your exploration of the Foothills until you stumble upon the broken wagon. Once uncovered, assign any of your party members to investigate – no battle ensues, sparing your entire party from involvement. A notification will prompt you to the exact location of the Bandit Camp, where Hildegard's husband is being held captive. Liberate him from the camp to successfully fulfill this objective. These are the crucial steps to unraveling the mystery of Hildegard's husband in For the King 2. If you found this guide beneficial, consider exploring our diverse range of other informative guides.](https://meropaper.com/wp-content/uploads/2024/01/for-the-king-2-hildegard-husband-cart2-150x150.webp)