Heat and Temperature | Physics grade XI :
Heat :
Heat is the form of energy which simulates our sense organs so that we feel hot or cold . It follows from a body of higher temperature to one of lower temperature .
Its unit is joule(J) in SI and is calorie in CGS .
1 calorie (cal) = 4.2 J
Temperature :
The degree of hotness or coldness of body is called temperature . It determines the direction of flow of heat .
Its unit is Kelvin(K) in SI and is degree Celsius (°C) in CGS .
Difference between heat and temperature :
| SN | Heat | SN | Temperature |
|---|---|---|---|
| 1 | It is the form of energy. | 1 | It is a degree of hotness and coldness of a body. |
| 2 | In, SI units, its unit is joule and in CGS system its unit is calorie. | 2 | Its SI unit is Kelvin and is degree celsius in CGS system. |
| 3 | It is a cause . | 3 | It is a effect. |
| 4 | Two bodies can be in thermal equilibrium without having same amount of heat . | 4 | Two bodies can’t be in thermal equilibrium if they are at different temperatures . |
| 5 | It is a measure of total kinetic energy of all molecules of a body. | 5 | It is measure of average kinetic energy of all molecules of the body. |
| 6 | Flow of heat is independent of amount of heat energy contained in the bodies in thermal contact . | 6 | Flow of heat dependent on temperature of two bodies.If flows from a body at higher temperature t a body at lower temperature. |
Thermal Equilibrium :
Two bodies are said to be in thermal equilibrium if they have same temperature.
Zeroth law of thermodynamics :
If two bodies are separately in thermal equilibrium with a third body,then the first two bodies must be in thermal equilibrium with each other . This is known as Zeroth law of thermodynamics .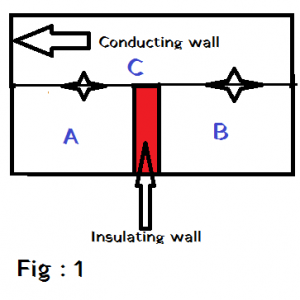
Consider three bodies A , B and C as shown in fig. 1 . Here A and C and also B and C are in thermal equilibrium . So A and B must be in thermal equilibrium with each other .
Lower Fixed Point (LFP) :
The temperature at which pure ice melts under standard atmospheric pressure is called Lower Fixed Point on a chosen scale.
Upper Fixed Point (UFP) :
The temperature at which pure water boils under standard atmospheric pressure is called Upper Fixed Point on a chosen scale .
Temperature Scale :
(i) Celsius Scale :
In this scale , LFP is 0°C and UFP is 100°C . The length between LFP and UFP is divide into 100 equal parts . Each part of division represents 1°C .
(ii) Farenheit Scale :
In this scale , LFP is 32°F and UFP is 212°F . The length between LFP and UFP is divide into 180 equal parts . Each part of division represents 1°F .
(iii) Kelvin/Absolute Scale :
In this scale , LFP is 273K and UFP is 373K . The length between LFP and UFP is divide into 100 equal parts . Each part of division represents 1K .
(iv) Reaumer scale :
In this scale , LFP is 0°R and UFP is 0°R . The length between LFP and UFP is divide into 80 equal parts . Each part of division represents 1°R .
Relation between temperature scales :
(C – 0) / (100 – 0) = (F – 32) / (212 – 32) = (K – 273) / (373 – 273)
i.e.
C / 100 = (F – 32) / 180 = (K – 273) / 100
Some useful links : Electrochemical Equivalent (z), Chemical Equivalent (E), their Relations and Faraday’s Constant


 - [Resolving the Adventure Not Found Error in For the King 2](#) - [Understanding the Purpose of the Hardwork Skill in For the King 2](#) Upon liberating the prisoner from the cart in The Resistance chapter, the world unfurls for exploration. Roam the area until you chance upon an overturned wagon distinct from the prisoner cart, nestled in the Foothills area of the map. Should the wagon remain elusive, lean on Vision Scrolls or Find Distance items, available in town shops, dropped by enemies, or carried by specific characters such as the Scholar. Employ these tools to meticulously scrutinize the Foothills. Continue your exploration of the Foothills until you stumble upon the broken wagon. Once uncovered, assign any of your party members to investigate – no battle ensues, sparing your entire party from involvement. A notification will prompt you to the exact location of the Bandit Camp, where Hildegard's husband is being held captive. Liberate him from the camp to successfully fulfill this objective. These are the crucial steps to unraveling the mystery of Hildegard's husband in For the King 2. If you found this guide beneficial, consider exploring our diverse range of other informative guides.](https://meropaper.com/wp-content/uploads/2024/01/for-the-king-2-hildegard-husband-cart2-150x150.webp)