note credit : shruti gautam

Notes of chemistry Chapter 1:
This law gives the relation between volume and temperature of gas at constant temperature. It states that at constant pressure the volume of given mass of gas is increased or decreased by 1/273 of its volume at o Degree rise in temperature . Thus, V0 be then according to the law volume of gas at 1 degree celious
=v0+1/273*V0
=v(1+1/273)
At 2degree celious
=V0+(1+2/273)
at T degree
let v1 and v2 be the volume of a gas at two different temperature t1 degree celcious and t2 degree celicious temperature respectively at constant pressure. We can write
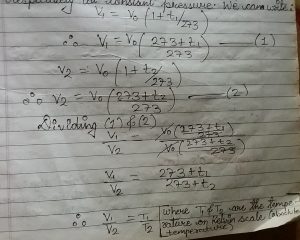
V1/T1= V2/t2
v/t= constant
V is directly proportinal to temperature
Hence charles law also state that at constant pressure the volume of given mass is directly porpotional to absloute temperature of gas.
Temperature Representation of charles law
Charle’s law can be Represented graphical by plotting volume of given mass of a gas against the temperature (on celsius scale) at constant pressure. A straight line is obtained which shows that the volume of gas at constant pressure is linear function of its temperature.
The temperature -273 is called absloute 0 degree temperature at that temperature the volume of all gases becomes 0.
Avogardro’s Law
Avogardro’s law states that “under similiar condition of temperature and pressure equall volumes of all gases contain equall number of molecules.”
Thus it follows that the volume of gases is directly proportional to the number of molecules at constant pressure and temperature.
V is directly proportional to n at Constant pressure and temperature.
Combined gas Equation
Boyle’s law and charles law gives seperately the effect of pressure and temperature on the volume of gas.
Combined gas equation gives simultance usuly about the effect of change of pressure and temperature on the volume of gas.
Let, The volume of mass of gas changed from V1 to V2 when pressure from p1 to p2 and temperature from t1 to t2 in following two types.
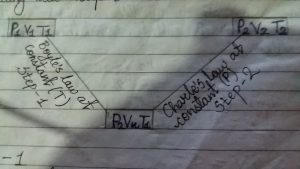
step 1
first suppose that volume of gas changes from v1 to vx and pressure from p1 to p2 at constant temperature “T”. Then according to Boyle’s law,
look at the picture for full equation

For 1 mole of gas the constant term k changed into R, which is also called universal gas constant.
PV=RT
Eq.2 is ideal gas equation for 1 mole of gas. For n mole of gas the ideal gas equation is
Pv= NRT
Nature of Gas constant(R)
From ideal gas equation PV=NRT
R=PV/NT
=Pressure * volume/no.of mole* absolute temperature
=force*volume/area*no.of mole*absolute temperature
=force*length/no.of mole *absolute temperature
=work/no.of mole* absolute temperature
Thus, R Represent workdone per mole per degree
SI unit of R= J mole-1k-1
R=8.314 J mol-1k-1
=0.0821 litre atom mole-1k-1
Ideal Gas equation in terms of density
Ideal Gas Equation can be changed in terms of density as follows ;
If “m” be the mass of gas “m” be the molecular weight of gas, then moles of gas.
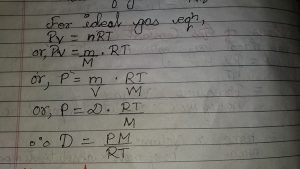
Important Questions of chemistry


 - [Resolving the Adventure Not Found Error in For the King 2](#) - [Understanding the Purpose of the Hardwork Skill in For the King 2](#) Upon liberating the prisoner from the cart in The Resistance chapter, the world unfurls for exploration. Roam the area until you chance upon an overturned wagon distinct from the prisoner cart, nestled in the Foothills area of the map. Should the wagon remain elusive, lean on Vision Scrolls or Find Distance items, available in town shops, dropped by enemies, or carried by specific characters such as the Scholar. Employ these tools to meticulously scrutinize the Foothills. Continue your exploration of the Foothills until you stumble upon the broken wagon. Once uncovered, assign any of your party members to investigate – no battle ensues, sparing your entire party from involvement. A notification will prompt you to the exact location of the Bandit Camp, where Hildegard's husband is being held captive. Liberate him from the camp to successfully fulfill this objective. These are the crucial steps to unraveling the mystery of Hildegard's husband in For the King 2. If you found this guide beneficial, consider exploring our diverse range of other informative guides.](https://meropaper.com/wp-content/uploads/2024/01/for-the-king-2-hildegard-husband-cart2-150x150.webp)